Introduction to the WHO-PQ Certification of SIDC
2025-10-20
保护视力色:
杏仁黄 秋叶褐
胭脂红 芥末绿 天蓝
雪青
灰 默认 【字体: 大 中 小 】
打印本页
关闭窗口
918博天堂
On October 28th, 2024, the Shanghai Institute for Drug Control (SIDC) was officially certified as a WHO-prequalified Medicines Quality Control Laboratory (WHO-PQ QCL) by the World Health Organization, and the certification was publicly announced on the WHO official website.
https://extranet.who.int/prequal/medicines/quality-control-labs/sifdc-drug-quality-control-center
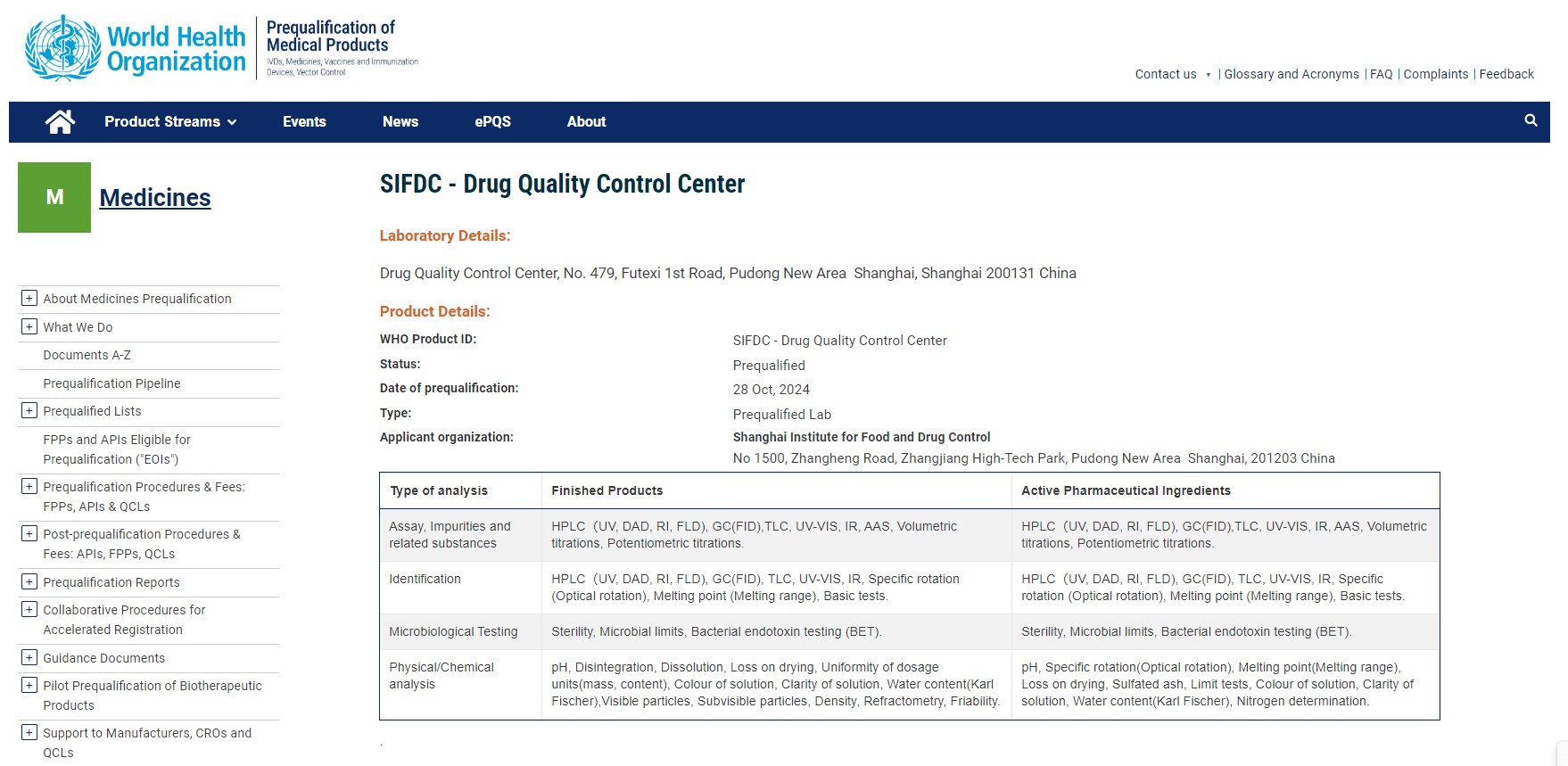
The Drug Quality Control Center, as an internal department and the technical implementation entity of SIDC, has enhanced the laboratory's capabilities in multiple aspects, including the quality management system, informatization development, software and hardware upgrades of instruments, and 3Q (IQ, OQ, and PQ) verification. The Drug Quality Control Center complies with the requirements of the WHO good practices for pharmaceutical quality control laboratories (WHO GPPQCL, WHO TRS 1052 Annex 4) and WHO good practices for pharmaceutical microbiology laboratories (WHO GPPML, WHO TRS 961 Annex 2). It covers the physicochemical and microbiological testing fields, constituting a Pharmaceutical quality control laboratory that fulfills the requirements of data integrity.
With the WHO-PQ certification, SIDC will provide testing services for medicines procured by the WHO and the United Nations, particularly those used to treat diseases such as AIDS, malaria, and tuberculosis. It will also offer internationally recognized testing and technical support for drug R&D, production, distribution, and regulation in the region, facilitating global mutual recognition of drug testing results and realizing the goal of "One test, Global Acceptance".